A simple method for raising the temperature in manual peptide synthesis
Wojciech Kamysz, Damian Neubauer
Department of Inorganic Chemistry, Faculty of Pharmacy, Medical University of Gdansk, Poland
Methods for obtaining synthetic peptides have undergone only a few changes over many years. Chemists do not like to modify synthesis protocols. This involves the need to change reagents and re-training employees. With the emergence of the Fmoc methodology, peptides have been obtained in the same way for over 30 years. After the success of microwave devices, many synthesizer manufacturers return to heating techniques in peptide synthesizers. The present research shows how to easily implement heating to the manual synthesis without the need to use water-heated vessels. Synthesis of three model peptides were performed using two reference methods (either the classic synthesis at room temperature or automatic synthesis on a microwave synthesizer) and two classic methods with dry heating blocks.
EQUIPMENT
The experiments were performed using either a Merrifield vessel for room temperature (method 1) and in the laboratory heating clamp (method 3) or else the Skiller 1 device at 60°C (heating block with a magnetic stirrer, method 4). Solid-phase reactors, heating clamp and the Skiller were provided by Lipopharm.pl, Zblewo, Poland.
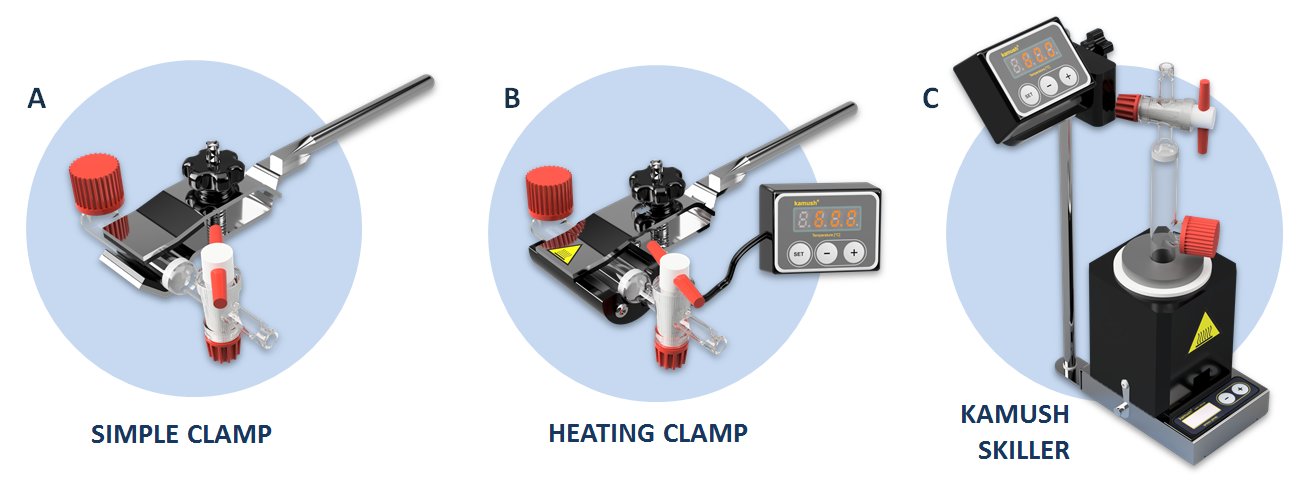
Figure 1. Manual synthesis equipment (A) simple clamp, (B) heating clamp, (C) Kamush Skiller 1.
The syntheses with the heating block were carried out in a glass vessel enabling thermostatting with air (the Kamysz vessel, 2×15 mL) for Skiller 1 and the classic Merrifiled vessel for laboratory clamp (20 mL).

Figure 2. Kamysz vessel (EUIPO nr 002753277-0001) and the method of its use in the synthesis with the device Skiller.
The synthesis with Skiller 1 and the heating clamp was performed at 60°C (temperature of the device setting). The actual temperature of the reaction mixture is shown in Figure 3.
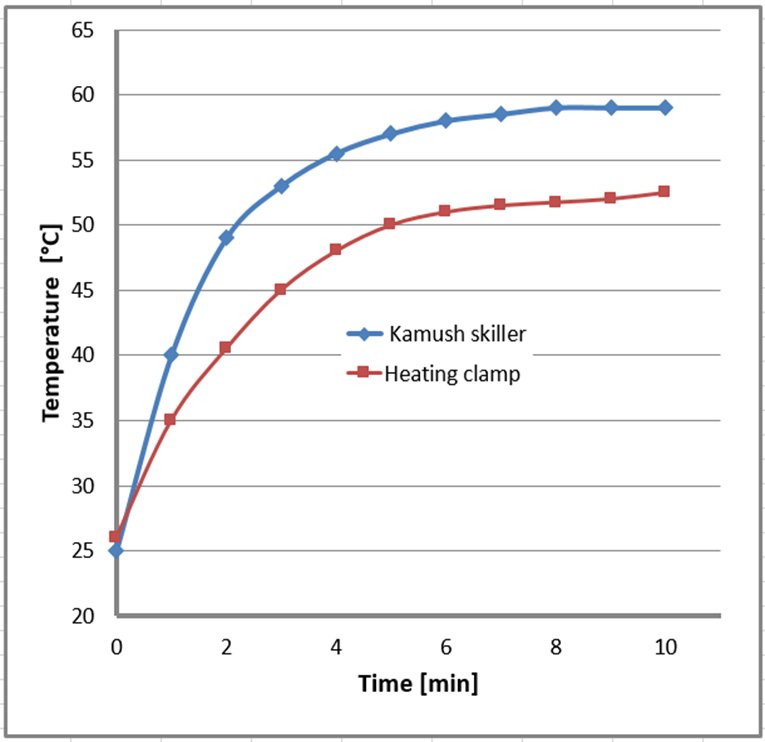
Figure 3. The efficiency of heat transfer through the heating clamp and Kamush Skiller 1. Vessels filled with 10 mL of DMF.
PEPTIDE SYNTHESIS PROTOCOL
The reference syntheses were carried out either at room temperature (classic synthesis) or following the synthesizer protocol. All peptides were synthesized using the Wang resin and a three fold excess of reagents (Fmoc-AA : DIC : OxymaPure, 1:1:1, mol/mol) dissolved in DMF based on the resin. Coupling was carried out for 2 h in the classic method and for 15 min when heated. Deprotection was performed with a 20% piperidine in DMF for 2 and 10 min in the classic method and for 2×2 min when heated. Synthesis with the use of a synthesizer (CEM) was carried out according to the manufacturer's procedure. The peptides were cleaved form the resin in TFA and a scavengers mixture (TFA : TIS : water : phenol, 94:2:2:2, v/v) for 1 h. The quantity of the crude peptides was determined and their purity was tested by HPLC.
The resins used in the experiment were examined independently for their mechanical degradation (Fig. 4).
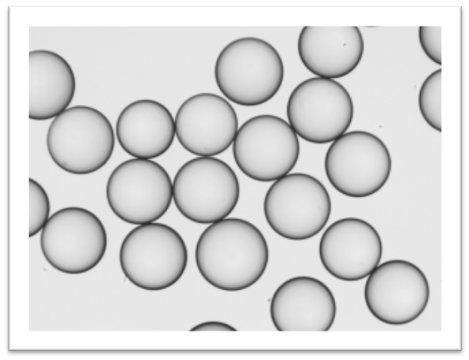
Figure 4. Microscopic image of the resin used on the Kamush Skiller taken after 10 cycles (Wang resin, www.peptideweb.com). Mixing with a magnetic stirrer at 300 rpm.
RESULTS
Elevated temperature increased efficiency of the synthesis to ensure peptide purity. Our preliminary study indicate that the heating clamp and the Skiller are simple alternatives to microwave synthesizer. However the synthesis with these blocks is manual and more time-consuming. On the other hand, these heating blocks are fully compatible with Fmoc chemistry, and not require additional equipment or training (are user-friendly), and occupy very little space. Moreover, these devices can be easily applied to a large scale synthesis. The results clearly indicate that the heating in dry heating blocks is an effective method to accelerate peptide synthesis.
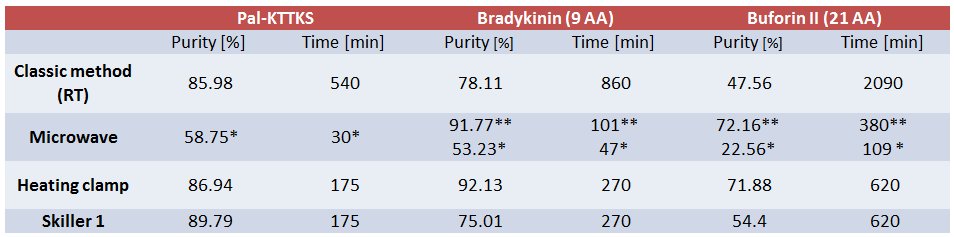
Purity was determined by RP-HPLC analysis. Method: 10-100%, 10 min, 1.2 mL/min, 214 nm (water-acetonitrile with 0.1% (v/v) TFA). Column: Waters X-Bridge 4.6 × 150 mm, 3.5 µm particle size, 130 Å pore size.
* Single standard coupling (15 sec at 75°C and 110 sec at 90°C);
** Double coupling of arginine, (25 min at 25°C and 2 min at 75°C) × 2.
REFERENCES
- Varanda, L. M.; Miranda, M. T. Solid-Phase Peptide Synthesis at Elevated Temperatures: A Search for and Optimized Synthesis Condition of Unsulfated Cholecystokinin-12. J. Pept. Res. 1997, 50 (2), 102–108.
- Bacsa, B.; Desai, B.; Dibó, G.; Kappe, C. O. Rapid Solid-Phase Peptide Synthesis Using Thermal and Controlled Microwave Irradiation. J. Pept. Sci. 2006, 12 (10), 633–638.


