FITC labeling
Fluorescein is well-known fluorescent dye used to label peptides and proteins. FITC excitation and emission wavelengths are 494 and 518 nm (green fluorescence), respectively [1]. In fact FITC has two isomers – fluorescein 5-isothiocyanate (FITC isomer I, CAS 3326-32-7; Figure 1) and fluorescein 6-isothiocyanate (FITC isomer II, CAS 18861-78-4; Figure 1). Furthermore, a mixture of isomers is also applicable (fluorescein 5(6)-isothiocyanate, CAS 27072-45-3).
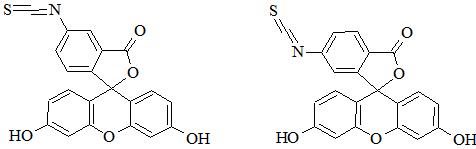
It is noteworthy that both isomers have almost identical fluorescence properties; however, conjugates can have different retention and electrophoretic behavior. Isothiocyanate can be easily conjugated with peptide/protein primary amine groups (e.g. lysine, ornithine, N-terminal amine). In SPPS fluorescent dye can be selectively introduced at N-terminal amino group. Unfortunately, this modification is usually associated with side reaction leading to the truncated peptide. This drawback is an effect of cyclization to fluorescent thiohydantoin with removal of the N-terminal amino acid. It can be overcome through additional spacer (e.g. β-alanine, 6-aminohexanoic acid) between FITC and N-terminal amino acid [2]. FITC can be also incorporated through conjugation of 5-IAF (5-(iodoacetamido)fluorescein, CAS 63368-54-7) with thiol group of cysteine. Moreover, FITC is used in FRET peptides and is usually paired with DABCYL as a quencher [3]. FITC can be attached to amino groups; however, to perform this reaction effectively it is necessary to provide appropriate pH at which amino group is deprotonated (deprotonated form prevails). Importantly, N-terminal amino group has lower pKa (8.9) than ε-amino group of lysine (10.5). Conjugation can be performed both on resin and in solution.
Protocol of conjugation of FITC to N-terminal amino group on resin
Reagents: DIPEA, DMF, FITC, peptide attached to resin (deprotected N-terminal amino group).
1. Fmoc group of the N-terminal amino acid must be removed. Use standard 20% piperidine in DMF (v/v) for 15 minutes with agitation. Afterwards, wash the resin with DMF and DCM and perform chloranil test. Consider using of β-Ala, 6-Ahx or Boc-Lys(Fmoc)-OH as N-terminal amino acid to avoid cyclization to thiohydantoin.
2. Prepare FITC reagent. Use at least 3 molar excess (3 eq.) based on the resin. Dissolve FITC in DMF and add DIPEA (6 eq).
3. Add mixture to the resin and incubate in the dark for 2 hours or more (overnight) at room temperature with agitation.
4. Remove solution and wash resin with DMF.
5. Perform standard peptide cleavage. FITC does not require any specific additive. FITC can undergo photobleaching and therefore it is recommended to protect FITC reagent and FITC-peptide/protein conjugates from light.
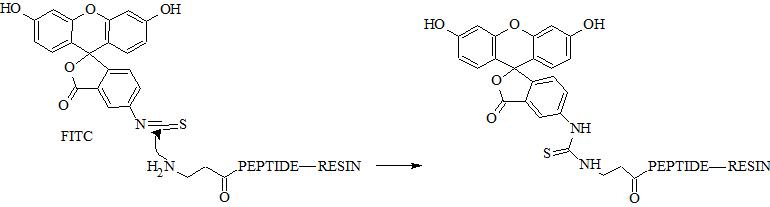
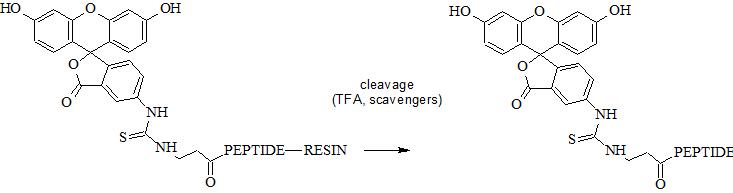
N-terminal amino group can be selectively conjugated with FITC in the presence of the side chain amino groups due to their different pKa values (approx. 8.9 and 10.5, respectively). Selective conjugation can be performed at specific pH range at which N-terminal amino group is deprotonated unlike ε-amino group. In fact pKa depend on peptide sequence and pH should be optimized experimentally. Reaction in solution usually require appropriate buffer.
Adjust pH near neutral if only N-terminal group should be engaged in conjugation. If all amino groups should react with FITC adjust pH above 9 (carbonate buffer). Protocol below describes alternative approach with DIPEA/TEA and organic solvents. Make sure that your peptide is soluble in proposed solution.
Protocol of conjugation of FITC to peptide amino groups in solution
Reagents: DIPEA/TEA, DMF/DMSO, FITC, peptide.
1. Dissolve peptide (approx. 1 mg/ml; 2 mM or less) and FITC (1.5-3 eq.) in DMF or DMSO.
2. Add TEA or DIPEA (25 eq.) and keep in dark for at least 4 hours. Reaction time can be extended. Control reaction progress using e.g. TLC.
3. Purify FITC-labelled peptide with HPLC or other technique. Remember to adjust pH of solution before purification to avoid damage to the HPLC system, especially to the column. Usually columns are stable within a specific pH range e.g. 2-8. Please verify this parameters in column specification. It is recommended to acidify your sample with acetic acid or trifluoroacetic acid.
Abbreviations
5-IAF – 5-(iodoacetamido)fluorescein6-Ahx – 6-aminohexanoic acid
Ala – alanine
β-Ala – Beta-alanine
Boc – tert-butyloxycarbonyl
DABCYL – 4-((4-(dimethylamino)phenyl)azo)benzoic acid
DCM – dichloromethane
DIPEA (DIEA) – N,N-diisopropylethylamine
DMF – N,N-dimethylformamide
DMSO – dimethyl sulfoxide
FITC – fluorescein isothiocyanate
Fmoc – 9-fluorenylmethoxycarbonyl
FRET – Förster resonance energy transfer
HPLC – high-performance liquid chromatography
Lys – lysine
SPPS – solid-phase peptide synthesis
TEA – triethylamine
TLC – thin-layer chromatography
References
[1] I. Johnson, M.T.Z. Spence, eds., The molecular probes handbook. A guide to fluorescent probes and labeling technologies, 11th ed., Life Technologies, 2010.
[2] M. Jullian, A. Hernandez, A. Maurras, K. Puget, M. Amblard, J. Martinez, G. Subra, N-terminus FITC labeling of peptides on solid support: the truth behind the spacer, Tetrahedron Lett. 50 (2009) 260–263. doi:10.1016/j.tetlet.2008.10.141.
[3] T.M. Feltrup, B.R. Singh, Development of a fluorescence internal quenching correction factor to correct botulinum neurotoxin type A endopeptidase kinetics using SNAPtide, Anal. Chem. 84 (2012) 10549–10553. doi:10.1021/ac302997n.


